CDC graphic from press conference; to enlarge, click on it.
—–
The Biden administration announced Wednesday that it will offer coronavirus booster vaccinations the week of Sept. 20, subject to authorization from the Food and Drug Administration.
The boosters are needed to address waning immunity as a result of the highly contagious Delta variant of the coronavirus. For now, it will apply only to the Pfizer BioNTech and Moderna vaccines, which initially required two doses; research on the Johnson & Johnson one-dose vaccine is not as complete.
Three studies published Wednesday by the
Centers for Disease Control and Prevention show declining effectiveness against infection by the virus after several months, but sustained and high effectiveness against hospitalization and death.
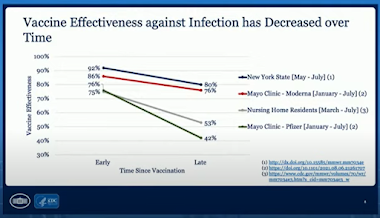
A New York state
study found a decline in vaccine effectiveness against infection between May and July, from 92% to 80%.
A
Mayo Clinic study between January and July found a decline in effectiveness against infection by the Delta variant in both vaccines: from 76% to 42% with the Pfizer vaccine and from 86% to 76% for the Moderna shot.
And from mid-February to Aug. 1, vaccine effectiveness against infection in residents in nursing homes
dropped from 75% before the Delta variant hit to 53% afterward.
“Around the six-month mark in the data, you start to see increases in mild to moderate infection,” Surgeon General Vivek Murthy said at a Wednesday
news briefing, adding that “the most important purpose of the vaccine is to keep us out of the hospital and to save our life.”
“We are seeing that still holding at a high level, which is good news,” Murthy continued. “But our anticipation is that if the trajectory that we are seeing continues, that we will likely see in the future an increase in breakthrough hospitalizations and breakthrough deaths.”
The booster shots will be available to fully vaccinated adults (18 years and older) who have received either the Pfizer or Moderna vaccine. These individuals will be eligible for these qualifying adults eight months after their second dose.
At this time, the Pfizer and Moderna booster shot is available for immunocompromised individuals and in Kentucky, and anyone living in long-term care facilities.
Murthy added that he anticipates that a booster will also be needed for those who received the Johnson & Johnson vaccine as more data becomes available.