Proposed new CDC guidelines for treating pain would encourage use of non-opioid therapies first, avoid one-size-fits-all approach
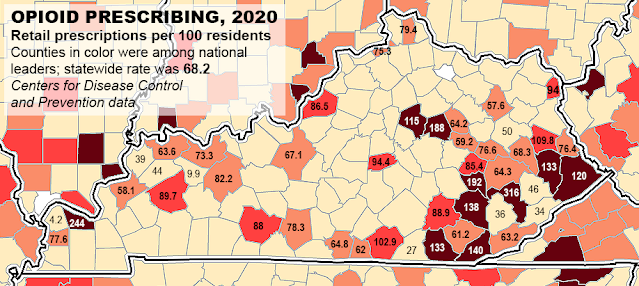
Centers for Disease Control and Prevention map, adapted by Ky. Health News, shows opioid-prescribing rates in counties that ranked highest and those surrounded or nearly surrounded by them.
—–
By Melissa Patrick
Kentucky Health News
The Centers for Disease Control and Prevention has proposed new guidelines that would move away from a one-size-fits-all approach to managing pain, to one that encourages doctors to use their best judgment when prescribing opioid painkillers and encourage them to use “non-opioid therapies” first.
The guidelines are especially important in Kentucky, a smaller-than-average state that ranks fifth in the nation for opioid prescriptions and saw an increase of 49% in overdose deaths in 2020, the last year reported.
In addition to prioritizing non-opioid therapies such as ibuprofen and other non-steroidal anti-inflammatory drugs, the proposed guidance drops previous recommended limits for dosing and encourages the use of immediate-release opioids, as opposed to long-acting ones, when possible.
It also offers extensive guidance for how to treat acute and chronic pain and how doctors should address patients who test positive for illicit substances.
“This clinical-practice guideline provides recommendations only,” the proposal says. “It does not replace clinical judgment and individualized, patient-centered decision-making.”
The guidelines, which are still in draft form, would update the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain, which were designed to slow the prescribing of opioids like OxyContin, which fueled the opioid epidemic.
Synthetic opioids like the rarely prescribed fentanyl and its analogues in the illegal drug supply now fuel most of the drug-overdose deaths in Kentucky, but the state ranks high in opioid prescriptions. Stroudwater Associates reported that Kentucky had the fifth highest opioid prescribing rate in the nation in 2020, with Perry, McCracken, Clark, Owsley, Whitley, Bell, Floyd, Pike, Clay and Fayette counties making up the top 10. Stroudwater is a health-care consultancy that says it focuses on rural and community hospitals, health-care systems and large physician groups.
The new CDC guidance was prompted by unintended consequences of the 2016 recommendations, which created barriers for care of people with chronic or severe pain, many of whom relied on opioid doses far higher than the recommended amount. The 2016 guidance also resulted in some people who no longer had access to these painkillers switching to heroin, and some physicians stopped caring for pain patients for fear of criminal and civil penalties.
The report on the guidelines adds that other misapplications of the 2016 rules included extension of them to patients not covered in the guidelines, like those with cancer or palliative care; the abrupt discontinuation of the drugs in some patients; duration limits by insurers and pharmacies; and patient dismissal and abandonment.
“These actions are not consistent with the 2016 CDC Guideline and have contributed to patient harm, including untreated and undertreated pain, serious withdrawal symptoms, worsening pain outcomes, psychological distress, overdose, and suicidal ideation and behavior,” the report says. It notes that many states have passed laws based on the 2016 guidance, even though the rules were meant to “support, not supplant, individualized, patient-centered care.”
The Kentucky General Assembly has passed several opioid-control laws in recent years, including one to limit opioid prescriptions for acute pain to a three-day supply, with exemptions.
The new guidelines recognize the dangers associated with opioids, but also recognize their value in treating pain. It offers 12 recommendations for clinicians who are prescribing opioids for adult outpatients with pain. They do not apply to patients suffering pain from cancer or sickle-cell disease, or those in end-of-life or palliative care.
The report says the recommendations are “based on a systematic review of the available scientific evidence while considering benefits and harms; patients’, caregivers’, and clinicians’ values and preferences; and resource allocation.”
The first guideline recommends that providers use non-opioid alternatives whenever possible, stating, “Clinicians should only consider opioid therapy for acute pain if benefits are anticipated to outweigh risks to the patient.”
Non-opioid treatments include things like over-the-counter medications like ibuprofen and acetaminophen; prescription medications like gabapentin; physical therapy; massage, and acupuncture.
After weighing the benefits and the risk of using an opioid to treat pain, the draft guidance says the provider is encouraged to start with the lowest effective dose and to prescribe them for only as long as the patient is experiencing “pain severe enough to require opioids.”
The report says, “It is imperative that people with pain receive the most appropriate and effective pain treatment with careful consideration of the benefits and risks of all treatment options.”
The CDC is seeking public comment on these changes through April 11. “This comment period provides another critical opportunity for diverse audiences to offer their perspective on the draft clinical practice guideline. We want to hear many voices from the public, including people living with pain and the health care providers who help their patients manage pain,” Christopher M. Jones, director for the National Center for Injury Prevention and Control, said in a news release.
He added, “The ultimate goal of this clinical practice guideline is to help people set and achieve their personal goals to reduce their pain and improve their function and quality of life. Getting feedback from the public is essential to achieving this goal.”
Submit your comments at https://www.federalregister.gov/public-inspection/2022-02802/proposed-2022-clinical-practice-guideline-for-prescribing-opioidsexternal icon.